When’s the last time you heard someone claiming to crave a warm, cozy IPA or an ice-cold glass of Bordeaux? Probably never. And while we don’t usually think about the reason why we like our beverages served at specific temps, a new study says the answer lies in the interplay between ethanol taste and temperature.
In an article published in journal “Matter” on Wednesday, researchers report that the variation of “ethanol-like” tastes between beverages is likely more due to the interaction between water and ethanol on a molecular scale rather than the ABV alone.
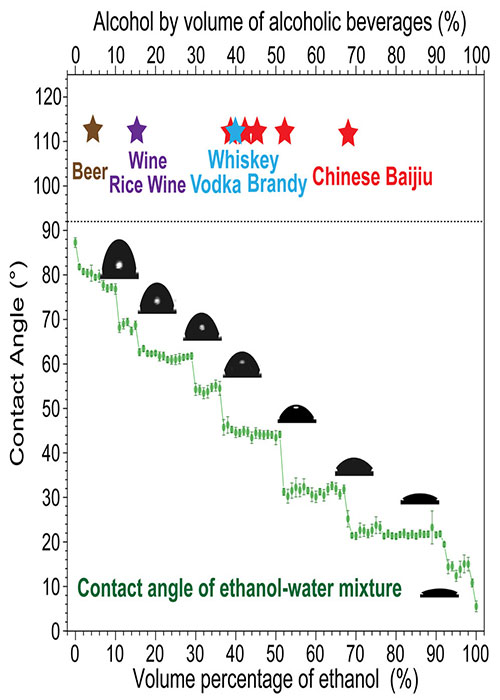
“I had the idea to ask the question ‘why does Chinese baijiu have a very particular concentration of alcohol, either 38-42 percent, 52-53 percent, or 68-75 percent?'” lead author and materials scientist of the Chinese Academy of Sciences Lei Jiang tells Phys.org of his reasoning behind the study. “So, I put a drop of beer on my hand to see the contact angle.”
The contact angle is a measurement of a given liquid’s surface tension, and is indicative of how the molecules within are interacting with one another and the surface they’re placed upon. For instance, water bears a low contact angle, so when a drop falls on a glass surface, it forms a bead-like shape. But if a high-ABV spirit is poured on glass, it will become flat and spread out, indicative of its high contact angle.
The research showed that this phenomenon is due to the formation of different molecular clusters — either chain-like or tetrahedral-shaped (like a pyramid) — in ethanol and water solutions. Those lower in ethanol formed tetrahedral structures around water molecules, and in the highly-concentrated solutions, the ethanol took to a chain-like fashion. In a nutshell, the chain-like molecular structures align with higher ethanol tastes (which drinkers tend to prefer), and the ethanol flavor tapers off when the molecules adhere to a tetrahedral shape.
Jiang, researcher Xiaotao Yang, and their team embarked on an experiment to measure the contact angle of a number of solutions with varying concentrations of alcohol in water. To their surprise, the contact angles did not increase in a linear fashion with higher alcohol concentration. Rather, their findings showed a series of plateaus.
The real kicker came when the researchers observed that the plateauing contact angles disappeared and reappeared in light of temperature changes in the varying solutions. These patterns could shed light on how the taste of ethanol is perceived on the palate.
“Influence of temperature on clusters probably accounts for preferable changes of ‘ethanol-like tastes’ of low-ABV beers or white wine after cooling and high-ABV shochu or Chinese baijiu after heating,” according to the research paper. To illustrate, varying ethanol solutions comparable to those is baijiu bear stark differences in molecular cluster shape at room temperature, but that discrepancy vanishes at higher temperatures. Therefore, two baijius with vastly different ABVs have a similar, harder-to-distinguish ethanol-like taste at high temperatures. “At low temperature, the tetrahedral clusters become the low concentration amount, and this is why we drink cold beer,” Jiang tells Phys.org.
Beverage companies can potentially use these findings to better capture an ethanol-like taste in their products with lower levels of ethanol concentration, allowing them to both increase profit margins and better market their products with suggested serving temperatures in mind — whether it’s ice-cold beer, chilled white wine, or hot sake.
